Chemist Interview Questions
A chemist is a professional who specializes in the study of chemistry, a branch of science that explores the properties, composition, and behaviour of matter. Chemists play a crucial role in various industries, including pharmaceuticals, petrochemicals, and materials science. They conduct experiments, analyses substances, and develop processes to create new materials or improve existing ones.
Whether working in research and development, quality control, or academia, chemists contribute to advancements in technology, medicine, and environmental sustainability. Their expertise extends to understanding molecular structures, reactions, and the practical applications of chemical principles in the pursuit of scientific innovation and problem-solving.
Basic Chemistry Interview Questions with Answers
Question: Can you explain the Periodic Table? why it is significant?
Answer: Periodic Table: A chart organizing elements based on atomic number (number of protons) and increasing atomic mass. Elements with similar properties are grouped together.
Rows (periods): Increasing electron shells
Columns (groups): Similar valence electron configurations and chemical properties
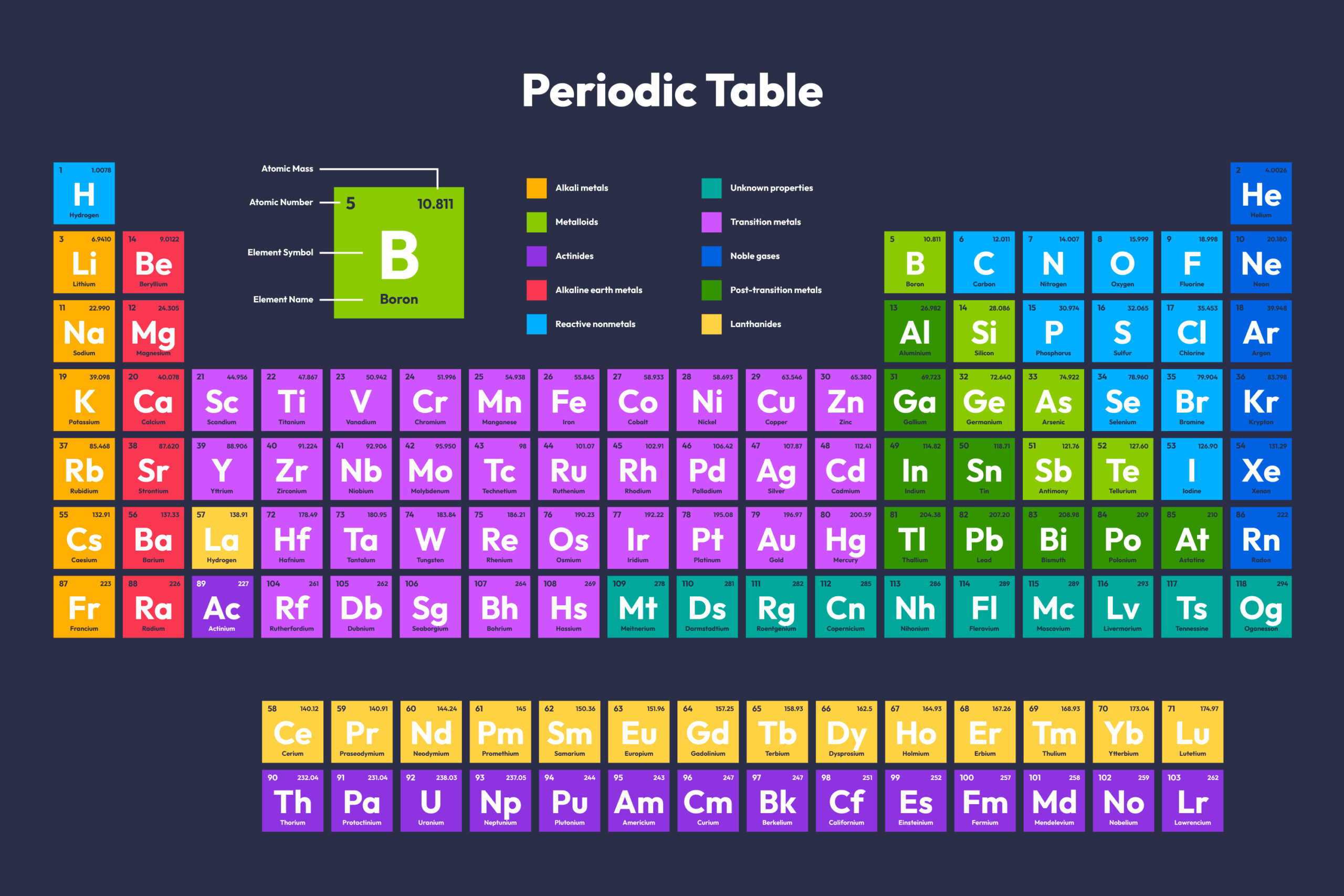
The Periodic Table: More Than Just a Chart:
Imagine a map that organizes all the building blocks of our universe! That’s what the periodic table is. It arranges elements (pure substances) based on their atomic number (number of protons) and reveals fascinating patterns about their properties.
Why is it significant?
- Explains why elements behave similarly within groups (columns) due to shared valence electrons (outermost electrons involved in bonding).
- Predicts properties of new elements based on their position in the table.
- Helped scientists discover new elements and develop new technologies.
Question: Give an example: Balance a simple chemical equation.
Answer: Think of a chemical reaction like a recipe. You need the right amount of ingredients to get the desired product. Balancing an equation shows the exact number of reactants needed to produce the desired number of products.
Example: Hydrogen (H2) + Oxygen (O2) -> Water (H2O)
Unbalanced: 2H + O -> H2O (missing an oxygen atom on the product side)
Balanced: 2H2 + O2 -> 2H2O (now every element has the same number of atoms on both sides)
Question: Can you describe the states of matter and their interconversions?
Answer: Matter can exist in different states:
- Solid: Fixed shape and volume (e.g., ice)
- Liquid: Definite volume but takes the shape of its container (e.g., water)
- Gas: Fills its container and has no definite shape or volume (e.g., air)
These states can change due to physical changes (no new substances formed) like:
- Melting: Solid to liquid (e.g., ice to water)
- Freezing: Liquid to solid (e.g., water to ice)
- Evaporation: Liquid to gas (e.g., water to vapor)
- Condensation: Gas to liquid (e.g., vapor to water droplets)
Remember: Chemical changes involve the formation of new substances, while physical changes involve just a change in state or appearance.
Question: Define the term ‘element’ and provide examples?
Answer:
Element: A pure substance made up of only one type of atom. All atoms of an element have the same number of protons. Examples: Hydrogen (H), Oxygen (O), Aluminum (Al).
Question: What is the difference between an atom and a molecule?
Answer:
Atom: The smallest unit of matter that can retain the properties of an element. It consists of a central nucleus (protons and neutrons) and electrons orbiting around it. Examples: Hydrogen (H), Oxygen (O), Iron (Fe).
Molecule: A group of two or more atoms held together by chemical bonds. Molecules are the smallest unit of a compound that retains its chemical properties. Examples: Water (H2O), Carbon dioxide (CO2), Glucose (C6H12O6).
Question: Can you define Valence Electrons?
Answer:
Valence electrons: The outermost electrons in an atom’s electron cloud, responsible for chemical bonding and determining an element’s reactivity. Examples: Helium (He) has 2 valence electrons (stable, unreactive), Chlorine (Cl) has 7 valence electrons (highly reactive).
Question: Can you define the terms ‘acid’ and ‘base’?
Answer:
Acid: A substance that donates a proton (H+) in a chemical reaction. Examples: Hydrochloric acid (HCl), Sulfuric acid (H2SO4).
Base: A substance that accepts a proton (H+) in a chemical reaction. Examples: Sodium hydroxide (NaOH), Calcium hydroxide (Ca(OH)2).
Question: What is the pH scale? How is it used to measure acidity and basicity?
Answer:
pH scale: A logarithmic scale ranging from 0 to 14, measuring the acidity or basicity of a solution.
pH < 7: Acidic
pH = 7: Neutral
pH > 7: Basic
Question: What is the difference between exothermic and endothermic reactions?
Answer:
Exothermic reaction: A reaction that releases energy to the surroundings (feels warm). Example: Burning wood.
Endothermic reaction: A reaction that absorbs energy from the surroundings (feels cool). Example: Dissolving ammonium chloride in water.
Question: Can you define oxidation and reduction reactions.
Answer:
Oxidation: Loss of electrons by an atom or molecule.
Reduction: Gain of electrons by an atom or molecule.
Redox reactions: Always involve both oxidation and reduction happening simultaneously.
Question: What is the difference between a physical change and a chemical change?
Answer:
Physical change: Changes the physical state or appearance of a substance, but not its chemical composition. Examples: Melting ice, dissolving sugar in water.
Chemical change: Changes the chemical composition of a substance, forming new substances with different properties. Examples: Rusting iron, burning paper.
Question: Can you explain the concept of stoichiometry?
Answer:
Stoichiometry: The quantitative relationship between reactants and products in a chemical reaction. It allows us to predict the number of products formed or reactants needed based on a balanced chemical equation.
Example: The balanced equation for the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O) is: 2H2 + O2 -> 2H2O. This tells us that 2 molecules of hydrogen react with 1 molecule of oxygen to produce 2 molecules of water. Stoichiometry allows us to calculate the amount of water produced if we have a specific amount of hydrogen or oxygen available, or vice versa.
Organic Chemistry Interview Questions and Answers
Question: What is an Organic Chemistry?
Answer: Organic chemistry is a branch of chemistry that studies the structure, properties, reactions, and synthesis of organic compounds, which contain carbon atoms. While carbon is the central element in organic compounds, these molecules often include hydrogen, oxygen, nitrogen, sulphur, and other elements as well. The field of organic chemistry is vast, as carbon forms a diverse range of compounds, including hydrocarbons and their derivatives.
Question: What are organic compounds, and how do they differ from inorganic compounds?
Answer:
Organic Compounds:
Main characteristic: Almost always contain carbon atoms, often bonded to hydrogen. This “carbon backbone” forms the core of many organic molecules.
Examples: Proteins, carbohydrates, fats, DNA, plastics, fuels, vitamins, medicines, etc.
Formation: Primarily produced by living organisms or through processes involving them.
Structure: Usually have more complex and diverse structures compared to inorganic molecules.
Bonds: Typically form covalent bonds, creating flexible and dynamic molecules.
Melting and boiling points: Generally, have lower melting and boiling points due to weaker intermolecular forces.
Reactivity: Can be quite reactive due to the presence of readily available electrons in their bonds.
Inorganic Compounds:
Main characteristic: Doesn’t necessarily contain carbon or carbon-hydrogen bonds. Instead, they involve a wide variety of elements and their interactions.
Examples: Water, salts, acids, metals, minerals, oxides, etc.
Formation: Naturally occurring through geological processes or synthesized in laboratories.
Structure: Often have simpler and more predictable structures.
Bonds: May involve ionic or covalent bonds, leading to a wider range of properties.
Melting and boiling points: Generally, have higher melting and boiling points due to stronger intermolecular forces.
Reactivity: Varies depending on the specific elements involved, ranging from highly reactive to inert.
Question: Can you define the term ‘functional group’ and provide examples?
Answer:
Functional Groups:
A functional group is a specific arrangement of atoms within a molecule that gives it characteristic chemical properties. They act like “molecular tags” influencing how the molecule behaves and interacts with other substances.
Examples:
Hydroxyl group (OH): Present in alcohols, phenols, and carbohydrates, makes them polar and able to form hydrogen bonds.
Carbonyl group (C=O): Found in aldehydes, ketones, and carboxylic acids, involved in many important reactions like oxidation and addition reactions.
Amino group (NH2): Present in amines and amino acids, contributes to their basic nature and ability to act as nucleophiles (electron donors).
Halogen group (F, Cl, Br, I): Attached to various organic molecules, impacting their reactivity and physical properties.
Question: What is the difference between saturated and unsaturated hydrocarbons?
Answer: Hydrocarbons are organic molecules composed only of carbon and hydrogen atoms. They can be classified as either:
Saturated hydrocarbons: Contain only single bonds between carbon atoms. These molecules are “saturated” with hydrogen, meaning they have the maximum number of hydrogen atoms possible for each carbon. Examples: methane, ethane, propane.
Unsaturated hydrocarbons: Have at least one double or triple bond between carbon atoms. These molecules are “unsaturated” because they could have additional hydrogen atoms if the double/triple bond were broken. Examples: ethene (double bond), ethyne (triple bond), benzene (aromatic ring with alternating single and double bonds).
Question: Can you explain isomers and their types?
Answer:
Isomers: Isomers are molecules with the same molecular formula (same number and types of atoms) but different structural formulas (arrangement of atoms). They are like different words made with the same letters.
Types of Isomers:
Structural isomers: Differ in the way their carbon atoms are bonded together. Examples: butane and 2-methylpropane (both C4H10).
Stereoisomers: Have the same connectivity but differ in the spatial arrangement of their atoms. Examples: cis- and trans-alkenes (differ in double bond geometry).
Enantiomers: Mirror-image isomers that are non-superimposable. Examples: L- and D-amino acids (differ in 3D structure).
Question: What is the isomerism?
Answer:
Isomerism is the phenomenon of molecules having the same molecular formula but different structures and properties. This can lead to significant differences in their physical and chemical behavior. For example, cis- and trans-fats have the same formula but different health effects due to their isomerism.
Question: Can you define the terms ‘alkane’, ‘alkene’, and ‘alkyne’?
Answer:
Alkane, Alkene, Alkyne: These are specific types of hydrocarbons distinguished by the type of carbon-carbon bonds they contain:
Alkane: Only single bonds between carbon atoms (saturated). Examples: methane, ethane, propane.
Alkene: Contains at least one double bond between carbon atoms (unsaturated). Examples: ethene, propene.
Alkyne: Contains at least one triple bond between carbon atoms (unsaturated). Examples: ethyne, propyne.
Question: What is a substitution reaction in organic chemistry?
Answer:
Substitution Reaction:
In a substitution reaction, an atom or group within a molecule is replaced by another atom or group. This often involves:
- Nucleophile: An electron-rich species attacking the molecule.
- Electrophile: An electron-deficient region within the molecule accepting the attack.
- Leaving group: The departing atom or group.
Examples: Alcohols reacting with acids (substitution of OH with halogen), alkyl halides reacting with nucleophiles (substitution of halogen).
Question: Define addition reaction and elimination reaction?
Answer:
Addition Reaction: Here, two or more molecules combine to form a single product. This typically involves:
Pi bonds: Double bonds breaking and forming new single bonds.
Electrophiles and nucleophiles: Similar roles as in substitution reactions.
Examples: Alkenes reacting with halogens (addition of halogen atoms), ketones reacting with Grignard reagents (addition of alkyl groups).
Elimination Reaction:
In this reaction, a molecule loses a small molecule (often water) resulting in a double bond or elimination of two adjacent groups. This usually involves:
Base: Accepts a proton (H+) from the molecule.
Leaving groups: Two atoms or groups eliminated together.
Examples: Dehydration of alcohols to form alkenes, dehydrohalogenation of alkyl halides to form alkenes.
Question: Predict the products of simple organic reactions (e.g., alcohols with acids)?
Answer: When an alcohol reacts with an acid, it typically undergoes an acid-catalyzed dehydration reaction. For example, the reaction of ethanol (CH₃CH₂OH) with sulfuric acid (H₂SO₄) can produce ethene (CH₂=CH₂) and water (H₂O).
Question: Describe a named organic reaction you studied (e.g., SN2)?
Answer: SN2 stands for “Substitution Nucleophilic Bimolecular.” It is a type of nucleophilic substitution reaction where a nucleophile attacks a substrate, leading to the substitution of one atom or group with another. The reaction involves a single concerted step.
Question: Explain the concept of stereochemistry and its importance?
Answer: Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules and the spatial relationships between different molecules. It includes concepts like chirality (handedness) and stereoisomerism, which can significantly impact the properties and reactivity of organic compounds.
Question: Explain the concept of resonance in organic chemistry?
Answer: Resonance describes the delocalization of electrons across multiple structures. This means a molecule cannot be represented by a single structure but rather a weighted average of several resonating structures. Resonance helps explain the stability and reactivity of certain molecules.
Question: What are carbohydrates, lipids, proteins, and nucleic acids, and what are their roles in living organisms?
Answer:
These are organic molecules vital for life:
Carbohydrates: Provide energy (sugars) and structural support (cellulose).
Lipids: Fats and oils; energy storage, insulation, and structural components of cell membranes.
Proteins: Amino acid chains; enzymes, structural components, and signalling molecules.
Nucleic Acids: DNA and RNA; store and transmit genetic information.
Question: What are polymers, and how are they formed?
Answer:
Polymers are giant molecules made up of repeating units called monomers. Imagine them like long chains built by connecting individual beads (monomers). They can be categorized as:
Natural polymers: Occur in nature, like proteins, DNA, cellulose (in plants), and rubber.
Synthetic polymers: Man-made, such as nylon, polyethylene, and Teflon.
Polymer formation happens through a process called polymerization. There are different types, but two main categories are:
Addition polymerization: Monomers with double bonds join together, breaking the double bond and forming single bonds to create a long chain. Example: polyethylene from ethylene.
Condensation polymerization: Monomers react, eliminating small molecules like water with each connection, forming the polymer chain. Example: nylon from diamines and dicarboxylic acids.
Various factors influence the properties of polymers, including:
Monomer type: Different monomers result in different polymer properties.
Polymerization method: The process affects chain length, branching, and structure.
Additives: Substances added during processing can modify properties like flexibility, color, and stability.
Question: Can you outline the steps involved in preparing a standard solution?
Answer:
The steps involved in preparing a standard solution include:
- Weighing a precise amount of the solute using an analytical balance.
- Transferring the solute to a volumetric flask using a funnel.
- Dissolving the solute in a small amount of solvent.
- Diluting the solution to the desired volume with the solvent.
- Inverting the flask to ensure thorough mixing.
Question: What is the difference between qualitative and quantitative analysis?
Answer: Both analyse samples, but differ in the information they provide:
Qualitative analysis: Determines the presence or absence of specific components in a sample. It identifies what elements or functional groups are present, not their exact amounts. Techniques used include color tests, precipitation reactions, and spectroscopy.
Quantitative analysis: Determines the amount or concentration of specific components in a sample. It measures the precise quantity of a specific element or compound present. Techniques used include titrations, gravimetric analysis, and instrumental methods like spectrophotometry or chromatography.
General Interview Questions with Sample Answers
Question: Why are you interested in pursuing a career in chemistry?
Sample answer: I’ve always been fascinated by the fundamental principles of how the world works at its most basic level. Chemistry offers a unique lens to explore these principles, understand the interactions between substances, and create new materials that impact various aspects of life. This combination of knowledge, problem-solving, and innovation deeply excites me and motivates me to pursue a career in this field.
Question: What do you know about the specific industry or company you’re applying to?
Sample answer: I’ve thoroughly researched your company’s mission, values, and recent breakthroughs in [mention specific area]. I’m particularly impressed by your commitment to [mention specific initiative] and believe my skills and experience in [mention relevant skills] align perfectly with your current projects in [mention specific area].
Question: Can you describe your understanding of relevant regulations and safety protocols.
Sample answer: I understand the importance of safety and adherence to regulations in the chemical industry. I’ve familiarized myself with key regulations like [mention specific regulations] and safety protocols like [mention specific protocols]. I’m eager to learn more about your company’s specific safety guidelines and demonstrate my commitment to a safe and responsible work environment.
Question: How do you stay updated with current advancements in chemistry research?
Sample answer: I actively follow industry publications like [mention publications], attend relevant conferences and webinars, and participate in online communities like [mention communities]. I also enjoy delving into research papers on [mention specific areas of interest] to stay on top of the latest advancements.
Question: Tell me about your experience with specific industry-related software or techniques?
Sample answer: I have experience working with software like [mention software] for [mention specific tasks], as well as utilizing techniques like [mention techniques] for [mention specific applications]. I’m a quick learner and eager to expand my knowledge and expertise in any software or techniques relevant to your company’s projects.
Below are some useful Topics for interview questions and answers related to pharmaceuticals, covering Pharma safety , Organic Chemistry, Chemist R&D ,Pharmacist ,Quality Control, and Quality Assurance roles.
- Pharma Safety Interview Questions and Answers
- Safety Officer Interview Questions And Answers
- Quality Control Interview Questions & Answers In Pharma
- Pharmacist Interview Questions And Answers
- Chemist Interview Questions and Answers (Production & R&D,QC,QA)
- Quality Assurance Interview Questions in Pharma Industry





